The implementation of the European regulation on medical devices has taken a major step forward with the adoption of a proposal by the European Commission to adapt its transitional provisions.
The amendment to the Medical Device Regulation modifies Article 120 to maintain the validity of certificates issued under the directives and allow marketing of corresponding devices until the end of 2027 or 2028, depending on their risk classification.
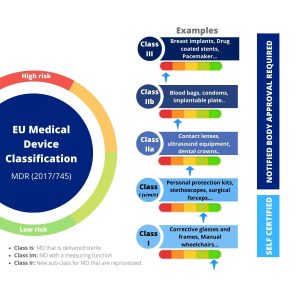
- Shorter transition period extension for high-risk devices, such as pacemakers and hip implants, until 31 December 2027.
- Longer transition period extension for medium- and low-risk devices, such as syringes or reusable surgical instruments: until 31 December 2028.
To benefit from this extension, companies must meet several conditions, including submitting technical documentation to a notified body before May 2024 and signing a contract before September 2024.
The regulatory amendment also repeals the end-of-market availability date originally scheduled for May 2025.
To learn More Visit: Link




