Webinar – Healthcare Actors: Are you GDPR compliant?

Participate in our live webinar “Healthcare actors: Are you GDPR compliant? ” hosted by our experts. Thursday, April 1, 2021 at 10am RGPD compliance is a crucial issue for healthcare actors. The 3-year deadline after the application of the European Regulation expires in May 2021. Will you be able to answer the question: “Are you […]
A 88/100 Gender Equality Index at Universal Medica For 2020
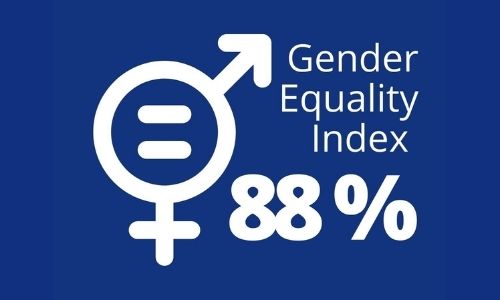
At Universal Medica, we are committed to gender equality at workplace. We have implemented robust actions to ensure true equality between men and women and absence of any discrimination within a company. The gender equality index is calculated within framework of the 5 September 2018 law on freedom of choice in professional careers places. The […]
Universal Medica Group supports the Movember movement for Men’s Health

The month of November is dedicated to moustaches and men’s health with the Movember movement. All Universal Medica Group employees have mobilised to support this event, which raises funds for research into men’s health issues. In France, prostate cancer is the most common, affecting more than 50,000 men a year and causing around 8,000 deaths. […]
All Universal Medica Group employees have mobilised in the fight against breast cancer

For October Rose, all Universal Medica Group employees have mobilised in the fight against breast cancer. Every year, nearly 58,000 cases and 12,000 deaths are reported in France (1). If detected at an early stage, breast cancer can be cured in 90% of cases (2). This is why, throughout this month, our teams have been […]
Webinar – Cosmetovigilance, Key points to implement a compliant system

Universal Medica’s vigilance experts led the webinar on the theme of Cosmetovigilance, organised by COSMED, which is the leading network representing SMEs in the French cosmetics industry. The implementation of a Cosmetovigilance system, monitoring the safety of cosmetic products after they are placed on the market, is an obligation for the person responsible for cosmetic […]
Universal Medica Group has taken steps to ensure business continuity in accordance with regulatory requirements.

Universal Medica Group has taken all necessary steps to ensure business continuity in accordance with regulatory requirements. Our teams are more than ever mobilised to maintain the quality of services provided to the healthcare industry, healthcare professionals and patients. Do not hesitate to contact us for any information: contact@universalmedica.com 01 41 12 27 77.
Webinar – Materiovigilance: Everything you need to know about the new European Regulation on Medical Devices (EU Regulation 2017/745)

Universal Medica have hosted its next live webinar on Friday 23 October 2020 at 2:30pm on the topic of medical device vigilance. Our experts presented the new European regulation on post-marketing surveillance of medical devices and addressed the following points: – How to set up post-market surveillance of medical devices and which data to monitor? […]
Webinar – Universal Medica Group presents its solutions in Pharmacovigilance

PHARMACOVIGILANCE,
7 key points for a successful pharmacovigilance inspection
On 18/06/2020 at 17h30 (30 minutes).
Pharmacovigilance is one of the cornerstones of a drug’s life. On a daily basis, it enables the monitoring and prevention of the risk of adverse effects resulting from their use. The announcement of a pharmacovigilance inspection is an important step in the life of a pharmaceutical laboratory…
Webinar – Universal Medica Group presents its solutions to stay connected

CRISES MANAGEMENT,
5 key steps to manage crisis management in the pharmaceutical industry*
On 25/06/2020 at 17H30 (30 minutes)
Pharmaceutical companies are confronted with crisis situations that can have major consequences in terms of image, reputation and relationships with their direct or indirect customers. Whatever the type of crisis to be managed (shortage, industrial accident, unexpected adverse reaction, health crisis…)
GDPR for life science follow the US’ data privacy step

On 5th November 2019, two representatives from California (Silicon Valley, USA) proposed the implementation of an ‘American version’ of GDPR. This proposed law, known as the Online Privacy Act, would have the same goal as the General Data Protection Regulation (GDPR): the protection of citizens. Universal Medica Group, through the concept we have developed ‘GDPR […]



